Terminally ill Limavady boy denied medicine
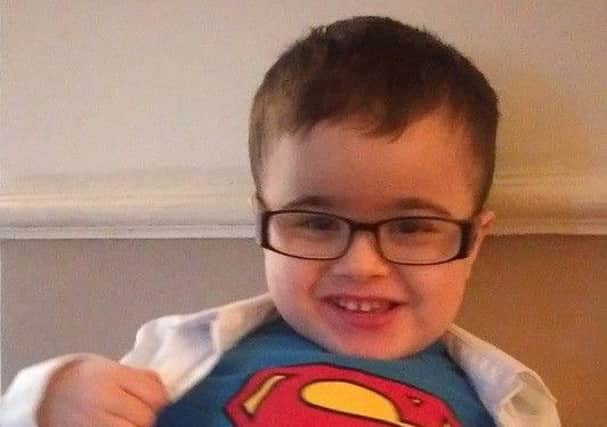

A campaign has been going on for some time to secure a new drug for Limavady boy Callum McCorriston, who suffers from an incurable form of muscular dystrophy known as Duchenne.
The condition will cause Callum’s muscles to waste away - meaning he will eventually lose the ability to walk and will likely die before he reaches his thirties.
Advertisement
Hide AdAdvertisement
Hide AdA ray of hope recently emerged, however, when a new drug known as Translarna showed that in some cases of Duchenne, the steady deterioration of the condition could be halted.
Callum is the only boy in Northern Ireland who would benefit from the drug but his mother’s application for the medicine to be made available to him has been turned down.
She said: “I have just received a call from Callums consultant to inform me that Callums application for Translarna has been denied.
“They have turned down a drug to help my baby stay ambulant (able to walk), stay active and protect his muscles.
Advertisement
Hide AdAdvertisement
Hide Ad“They have denied him his basic human right, the right to live. I am totally disgusted with their decision.
“I will not accept this answer and will do anything I can to get him what he needs.
“My heart is broke and my hope is fading, but I’ll fight for Callum.”
A spokesperson for the Health and Social Care Board said: “It is not appropriate for the Health and Social Care (HSC) Board to comment on the circumstances of an individual patient case.
Advertisement
Hide AdAdvertisement
Hide Ad“In considering a request for specialist drug treatment on behalf of an individual patient, the HSC Board must take account of the evidence of the drug’s effectiveness and, in this regard, applies NICE guidance where it is available. The Board also takes account of guidance from other national bodies, for example the Scottish Medicines Consortium (SMC), particularly if NICE guidance has not been issued.
“For Translarna, advice in regard to its cost effectiveness has not yet been issued by NICE or the SMC. In this circumstance Translarna is not currently commissioned in Northern Ireland.
“The HSC Board does, however, have a clear process by which individual patient requests can be considered. If the patient’s clinical circumstances are identified as exceptional, funding for the requested treatment can be approved by the Board.
“As part of that process the patient’s hospital consultant puts in writing the exceptional clinical circumstances which apply to a particular patient. This information is expected to demonstrate why that patient would be more likely than others with the same condition to benefit from the proposed treatment. Such requests require to be supported by nominated senior clinicians and managers within the relevant HSC Trust.
Advertisement
Hide AdAdvertisement
Hide Ad“It should be emphasised that cost is not the determining factor in reaching a decision regarding the appropriateness of an individual request for a specialist drug. Rather, the priority is to consider the evidence to support the drug’s use and the extent to which an individual may benefit from it.”